- Polystyrene exhibits co-solvency with acetone and alkanes at room temperature. However, to find the correct composition, assume that we have used acetone: hexane solvent mixtures as 20:80, 50:50, and 80:20. An osmotic pressure technique was employed on these systems at room temperature (298K) and the data obtained was given below. The solvent (mixture) density varies as 0.666 g/cm3, 0.7057 g/cm3 and 0.8062 g/cm3 and for 20:80, 50:50, and 80:20 mixtures.
Concentration versus osmotic pressure data for polystyrene with different
ratios of solvent mixture
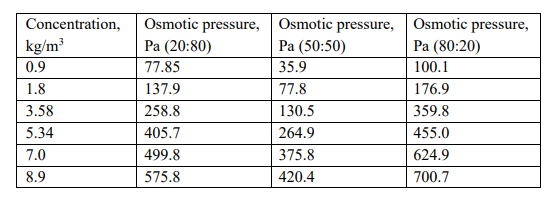
- Determine the molecular weight of the polymer and the second virial coefficient (A2) in each mixture of solvents. Assume that A3 and subsequent coefficients are negligible.
- Based on the obtained data, which solvent mixture is a good solvent for
polystyrene? Explain. - Looking at the molecular weights, A2 values, and osmotic data, are the obtained results reasonable? Justify your choice with proper reasoning.
- Are these ideal or non-ideal solutions? Explain.
Use the dataset of polystyrene provided in the table below and:
- Calculate molecular weight, A2, and radius of gyration of the polymer by preparing a Zimm plot.
- Explain the obtained data.
- What is the importance of extrapolation of data to small angles and zero
concentrations?
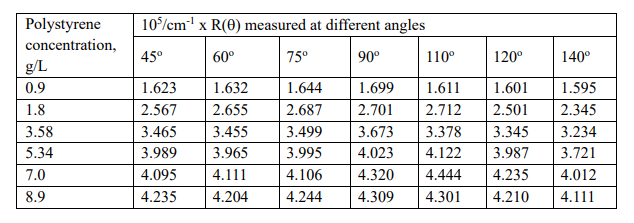
We will use a 660 nm wavelength light (l or l0) for this problem.
dn/dc = 0.10 mL/g
Rayleigh ratio for the solvent used: 1.207 x 10-3
/m
Refractive index for the solvent: 1.4898.
K – optical constant (depends on the refractive index, no, of the pure solvent)
c or c2 – polymer concentration
k (used in the plot) – arbitrary mathematical constant added to provide spacing between
curves (no physical meaning). For consistency, let us take it as ‘75’ in this question.
R(q) or DR = Rayleigh’s ratio