Question 1:
- For each of the following organometallic species calculate the oxidation state of the metal center, the total number of valence electrons, and give a diagram that represents the three-dimensional shape.
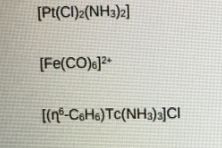
- By considering the extent of back-bonding in the following organometallic carbonyl complexes, which would have the longer C=O bond length. Explain your answer using diagrams and give reference to v CO.

- Element-element multiple bonds are less common in heavy element main group compounds. Describe synthetic strategies to overcome this.
Hire a Professional Essay & Assignment Writer for completing your Academic Assessments
+44-755-536-9184 Chat Now
Question 2:
- Explain by lanthanide ions exhibit lines like absorbance and emission spectra in comparison to d-block complexes which have broad spectra.
- What is the valence electron configuration of the Nd (III) ion?
- Calculate the magnetic moment of Nd (111) using the Lande formula below.
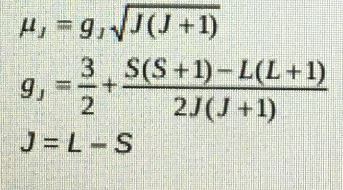
- Draw a labeled Jablonski diagram showing how a lanthanide excited state is populated.
- Give with examples, two uses of lanthanide complexes, explain your choice of metal and coordination environment.